Dear Customer
Dechra Veterinary Products Ltd is recalling stock of the product in the table below. The reason for the recall relates to the stability failure of the product which has been attributed to the excessive time that passed between the initial stage of the blend and the final compression stages of the manufacturing process during rework. The recall is for a singular batch only.
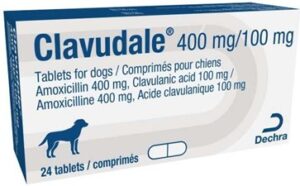
| NVS Product Code | Product Description | Affected Batch Number | Expiry Date |
| 789355 | Clavudale 400 mg /100mg Tablets, PK24 – VM 10434/4053 | R001 | 03/2024 |
What to do next – Please examine your stock immediately and take the following steps.
I have the affected product
- Quarantine the product.
- If you have any of the affected stock, then please contact our Customer Services Team on 01782 771111 (Option 3) to arrange the return of the products.
- This affected product will only be accepted until 23rd October 2023, after this date we will not accept this product.
I do not have this product
- Unless you’ve received email notification that our records indicate you may have this product, no further action is required.
If you require any further information, please contact our Customer Service Department on 01782 771 111.
Kind regards,
Andrew Duxbury
(Responsible Person)





